Research, Therapy, Clinical & Translational Research, Treatment, Article
Which PRF preparation offers most potential for osteogenesis?
11 July 2024
The EFP recently awarded its Graduate Research Prize 2023 for original graduate research. In the second of a series of articles by the four winners, Konstantinos Kosmidis (Academic Centre for Dentistry Amsterdam, The Netherlands) outlines the findings of a study of the osteogenesis potential of three different PRF preparations.
Periodontal disease often results in the loss of alveolar bone support, leading to periodontal osseous lesions. These lesions are significant because they indicate the loss of tooth support and highlight local factors such as deep pockets and furcation involvement that may signal disease progression. The surgical regeneration of the periodontium can offer various benefits including increased periodontal attachment, decreased pocket depth, and minimal gingival recession.
Successful periodontal tissue regeneration requires stimulating various cell types, particularly osteoblasts and osteoclasts, which are essential in bone remodelling. Platelet-rich fibrin (PRF) is a key material in guiding cells to promote tissue healing. PRF contains living cells such as leukocytes, platelets, and stem cells, which release growth factors and adhesion molecules that are crucial for tissue repair.
Initially developed with high relative centrifugation force (RCF) to form a dense fibrin clot, standard PRF (L-PRF) therapy has evolved in recent years. Today we can also offer:
- Advanced PRF (A-PRF+), which uses a lower RCF, resulting in a more porous matrix.
- Injectable PRF (i-PRF), which remains in liquid form, allowing it to be mixed with other biomaterials.
These PRF variants contain high levels of growth factors such as platelet-derived growth factor AA (PDGF-AA), epidermal growth factor (EGF), and insulin-like growth factor 1 (IGF-1), which accelerate wound healing and cell growth.
The whole idea started as a continuation of the research previously conducted by my colleague Luciano Pitzurra in our department at ACTA university. In this preclinical research, the advantages of A-PRF+ were shown in comparison with the classic L-PRF in the healing of fibroblasts. Since the periodontium is a complex structure composed of epithelial cells, periodontal ligament cells, and bone cells (osteoblasts, osteoclasts, and osteocytes), it was interesting to investigate the effect and possible advantages of A-PRF also on osteoblasts.
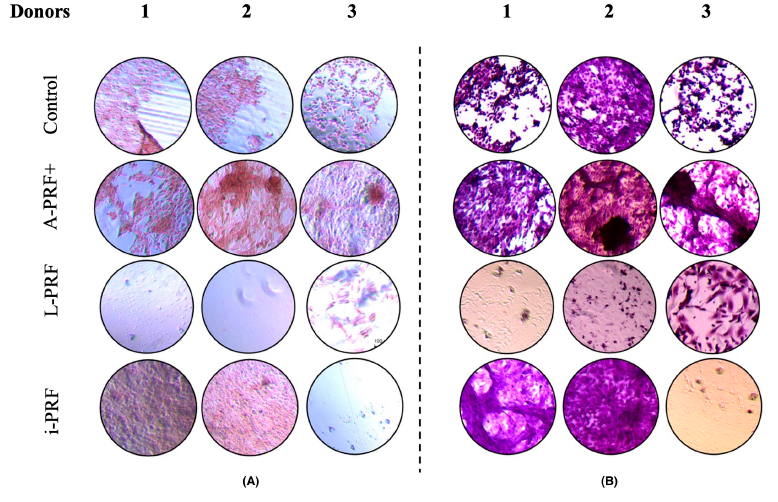
We took blood samples from six healthy male volunteers, which were used to prepare A-PRF+, L-PRF, and i-PRF following specific centrifugation protocols. The PRF membranes and supernatants were incubated with osteoblast-like cells. Ribonucleic acid (RNA) extraction, real-time quantitative polymerase chain reaction (qPCR), calcium assays, alkaline phosphatase activity, and mineralisation were conducted to measure gene expression, calcium content, enzyme activity, and mineralisation.
There were five main findings:
- Gene expression: Runt-related transcription factor 2 (RUNX2) expression was higher in the control group than in the A-PRF+ and L-PRF groups. Osteocalcin (OC) and intercellular adhesion molecule 1 (ICAM-1) showed significant upregulation in PRF conditions, indicating osteoblast differentiation. Collagen 1a (Col-1A) and alkaline phosphatase (ALP) did not show significant differences between conditions.
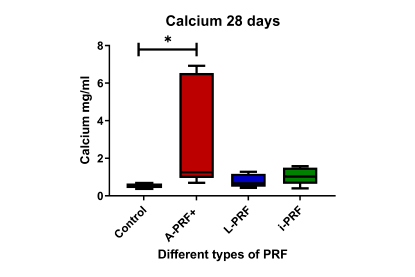
- Calcium deposition: Cells cultured with A-PRF+-conditioned medium showed significantly higher calcium production compared to the control, indicating enhanced mineralisation.
- Alkaline phosphatase activity: i-PRF significantly increased ALP activity compared to the control and A-PRF+ groups, suggesting enhanced enzymatic activity conducive to mineralisation.
- Cell differentiation and proliferation: i-PRF-conditioned medium caused more differentiation, but not higher proliferation of osteoblasts compared to other PRF conditions. No significant differences in proliferation rates were observed between different conditions.
- Mineralisation: A-PRF+-conditioned medium resulted in more mineralisation, indicating its potential for promoting bone formation.
The study found that A-PRF+ enhances mineralisation, making it potentially suitable for clinical applications requiring hard-tissue regeneration, while i-PRF promotes early differentiation but not proliferation. The study highlights the complexity of interpreting these results and suggests further research with newer PRF preparation techniques and clinical studies to determine their practical relevance.
PRF preparations can enhance the osteogenic potential of osteoblast-like cells, with A-PRF+ showing the highest potential for bone mineralisation. Further clinical studies are needed to confirm the efficacy of different PRF preparations in periodontal regeneration.
Bibliography
Choukroun J, Adda F, Schoeffler C, Vervelle A. Une opportunité en paro-implantologie: le PRF. Implant Dent. 2001;42(55):e62.´
Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e37-e44.
Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e45-e50.
Dohan DM, Choukroun J, Diss A, et al. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101(3):e51-e55.
Ghanaati S, Booms P, Orlowska A, et al. Advanced platelet-rich fibrin: a new concept for cell-based tissue engineering by means of inflammatory cells. J Oral Implantol. 2014;40(6):679-689.
Kobayashi E, Fluckiger L, Fujioka-Kobayashi M, et al. Comparative release of growth factors from PRP, PRF, and advanced-PRF. Clin Oral Investig. 2016;20:2353-2360.
Miron RJ, Pinto NR, Quirynen M, Ghanaati S. Standardization of relative centrifugal forces in studies related to platelet-rich fibrin. J Periodontol. 2019;90(8):817-820.
Pitzurra L, Jansen IDC, de Vries TJ, Hoogenkamp MA, Loos BG. Effects of L-PRF and A-PRF+ on periodontal fibroblasts in in vitro wound healing experiments. J Periodontal Res. 2020;55(2):287-295.

Biography
Konstantinos Kosmidis graduated from the Dental School of Athens in 2015 and, after three years as a general dentist, pursued specialisation at the EFP-accredited ACTA dental school in Amsterdam, starting in 2018. His research focused on platelet concentrates (PRF) for tissue regeneration. Since completing the specialisation in 2021, he has worked as a specialist dentist in periodontology and implant dentistry, participating in hands-on courses and pursing a keen interest in periodontology, implant dentistry, and regeneration research.




